Which Halogen Is Most Likely to React
Presence of electron withdrawing group such as NO 2. Fluorine is more reactive.
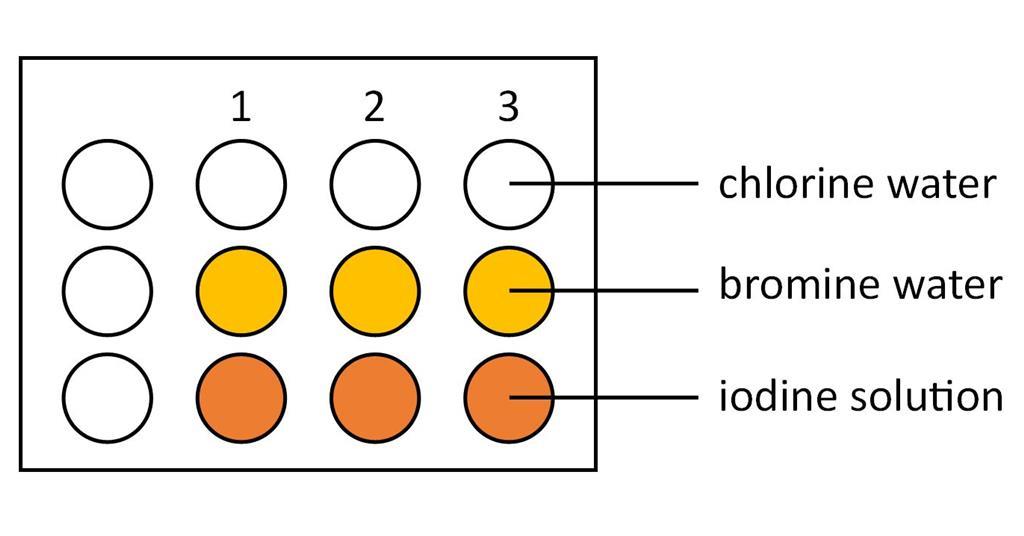
Halogens In Aqueous Solution And Their Displacement Reactions Experiment Rsc Education
Chlorine and bromine produce iron III chloride or bromide but iodine produces iron II iodide.

. F Cl Br I. CHO etc decreases the electron density on benzene. Which halogen is most likely to react.
This reactivity is due to high electronegativity and high effective nuclear. The principal oxidation states of chlorine. Which Halogen in group 7A is the most reactive.
Which halogen is most likely to react. Correct option is C Nucleophile always attacks electron deficient site. For each part question read the information and complete the answer lines below.
The fall in reactivity is because the halogens become less good oxidising agents as you go. The halogens are likely to react with essentially all elements with the exception of the inert gases. I When concentrated sulfuric acid.
Q- Why is Fluorine more reactive than other halogens. However like all blanket statements some caveats exist. There are two common forms of phosphorus which.
In organic chemistry free-radical halogenation is a type of halogenationThis chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of UV lightThe reaction is. Hence fluorine is the. The reactivity of the.
The reactivity of halogens decreases down the group which means Fluorine is the most reactive halogens as it is the most electronegative elements among the halogens and also the Periodic Table. Log in for more information. The Group I red and Group II tan elements can easily lose electrons during a reaction.
Fluorine exhibits the oxidation states of 1 F ion and 1 hypofluorous acid. Flourine Chlorine and Iodine are halogens and they react with. Cl chlorine Get the answers you need now.
Which element in group 1a is the most reactive. All halogens possess the oxidation state 0 in their diatomic forms. Correct answer to the question Which halogen is most likely to react.
This is because the valencebonding electrons are closer to the nucleus in Fluorine than they. Hydrogen reacts with fluorine. Halogens are highly reactive and they can be harmful or lethal to biological organisms in sufficient quantities.
Updated 3212017 113025 AM. Elements of other groups are much more likely to accept electrons as they react. The order of reactivity among Halogens from the more reactive least deactivating substituent to the least reactive most deactivating substituent halogen is.
The formation of trihalides PX3. 3 a In this question K L and M refer to a halogen atom or halide ion. Out of the four halogen elements Fluorine requires the least amount of energy for fission as F2 is extremely unstable due to high repulsion of electrons and small size.
The halogens are extremely. 1 on a question Which halogen is most likely to react. Asked 3112009 101627 PM.
The Hydrogen Halides HX The hydrogen halides are compounds that contain hydrogen attached to one of. All of the halogens react with phosphorus to give in the first instance phosphorus III halides - PX 3. The chemistry of the halogens is dominated by oxidation-reduction reactions.
Noble gases - do not usually react with other elements to form compounds How do the chemical properties of the halogens compare to those of the noble gases. All the halogens react directly with hydrogen forming covalent bonds andat sufficient levels of puritycolorless gases at room temperature.

Question Video Identifying Which Halide Ions Will Be Displaced From Solution By Elemental Fluorine Nagwa

No comments for "Which Halogen Is Most Likely to React"
Post a Comment